Unit 11+12
Intermolecular Forces and Solids
States of Matter
The fundamental difference between states of matter is the strength of the intermolecular forces. When the intermolecular forces are strongest, the substance is a solid. When the intermolecular forces are extremely weak, the substance is a gas.
Intermolecular Forces
Intermolecular forces are forces between molecules. They are not nearly as strong as the bonds that hold compounds together (called intramolecular forces).
Intermolecular forces dictate boiling points, melting points, surface tension, and many other physical properties.
Types of intermolecular forces
In order from weakest to strongest:
- London dispersion forces
- A nonpolar particle can be temporarily polarized, when more electrons are on one side than the other side.
- The tendency of an electron cloud to distort like this is called its polarizability.
- If a substance has more electrons, it has a higher chance of an uneven distribution. This means that it is "more polarizable" (has a higher polarizability), and therefore will have stronger intermolecular forces.
- More linear molecules have higher dispersion forces, because there is more surface area to interact with other molecules' forces.
- Dipole-dipole forces
- These are interactions between polar molecules.
- The oppositely charged ends attract each other.
- More polar molecules
- Hydrogen bonding
- Special dipole-dipole force that is much stronger.
- When H is bonded to N, O, or F (very electronegative elements), the other molecule pulls strongly on H's single electron. Therefore, H can make a very strong dipole-dipole bond with another N, O, or F atom on another molecule, called a Hydrogen bond.
- Ion-dipole forces
- Found in solutions of ions.
- The strength of these forces is what makes it possible for ionic substances to dissolve in polar solvents (such as dissolved in ).
Which intermolecular force has the greater effect?
If two molecules are of comparable size and shape, dipole-dipole interactions will likely be the dominating force.
If one molecule is much larger (molar mass close to double the size), London dispersion forces will likely determine its physical properties.
Phase Changes
A phase change is the conversion from one state of matter to another.
- melting/freezing
- vaporizing/condensing
- subliming/depositing
Vapor pressure
With a liquid sample, there is a minimum kinetic energy level required for a molecule to escape the sample and become a vapor. Although the average kinetic energy is below this, some molecules are above this level, and can escape into the space above the liquid.
These molecules in the air are sometimes recaptured by the liquid, which cycles molecules constantly. In an open container, molecules can drift away, so less molecules are recaptured. In humid conditions, more water vapor in the air causes water particles to bump back into the water more often, leading to more recapturing by the liquid water.
These molecules exert a pressure on the sample called vapor pressure.
The vapor pressure is higher when the sample is at a higher temperature, because more molecules will have sufficient kinetic energy to evaporate and join the molecules above the liquid (more molecules above = higher force pushing down on the liquid).
Boiling
When the vapor pressure equals the external pressure, the liquid boils. At this point, the vapor pressure pushes against the atmospheric pressure above with equal force, allowing molecules within the liquid to also escape since they aren't pushed down anymore. This causes the bubbles you see when a liquid boils.
This also means that the boiling point of a liquid depends on atmospheric pressure, so boiling points change with altitude.
Phase Diagrams
A phase diagram is a graph of pressure vs. temperature for a substance.
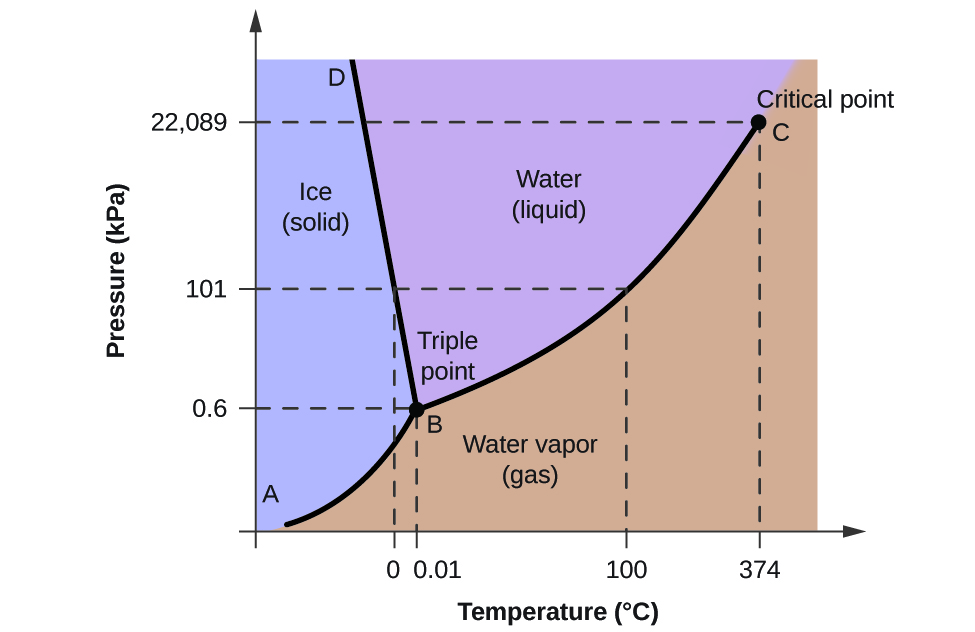
- Triple point - point where three states of matter intersect
- Critical point - intersection of critical temperature and critical pressure
- critical temperature - temperature beyond which a gas cannot be compressed
- critical pressure - pressure needed to liquify a gas at its critical temperature
Types of Solids
There are four types of solids (ranked* from weakest to strongest bonds): molecular solids, metallic solids, ionic solids, and network covalent solids.
*This ranking is approximate — properties vary, for example the ordering between metallic solids and ionic solids is not definite.
Therefore, higher boiling point = stronger intermolecular bonds.
Molecular solids
Consist of atoms or molecules held together by weaker intermolecular forces (London dispersion, dipole-dipole, or hydrogen bonds).
The shape matters for some physical properties.
Metallic solids
Since metals have few valence electrons and they're on high levels, the ionization energy is very low. This allows the electrons to form a "sea of electrons" around the cations (metals, but they don't have their electrons).
This model allows atoms to slide past each other without breaking.
Alloys
An alloy is a material that contains more than one metal and has beneficial properties.
A substitutional alloy involves atoms of one metal replacing atoms of another metal. This is only possible if atoms of the two molecules are similar in size.
An interstitial alloy involves the atoms of a smaller metal occupying positions in the "holes" between the atoms of a larger metal. This generally makes the alloy less ductile, since the smaller atoms create covalent bonds with neighboring atoms.
Ionic solids
The solid is comprised of alternatively charged ions.
Most favorable structures have cation-anion distances as close as possible, but cation-cation (repelling) distances are as far as possible.
These are quite hard but brittle. They're strong, but they're hit hard enough and ions shift, the repelling ions are facing each other and the entire substance shatters.
When identifying ionic solids, look for substances with a cation and an anion.
Network covalent solids
Atoms are covalently bonded over a large network with regular patterns of atoms.
Examples: diamond and graphite, silicon, quartz ().
Diamond vs. graphite
Diamond forms a tetrahedral shape ( hybridization), while graphite forms sheets of hexagonal shapes ( hybridization) that are held together by London dispersion forces.
Both are made of carbon only, but since graphite has the weaker intermolecular bonds, it's weaker and comes apart in sheets (such as when writing with a pencil).
However, this means that graphite is a conductor, since each carbon has a free electron (it only made 3 bonds) that can move and conduct charges.